Introduction
Overnight pulse oximetry (SpO2) studies are often used to qualify patients for long-term oxygen therapy or screen an individual for oxygen desaturations that may be associated with obstructive sleep apnea. Regardless of the specific application of continuous pulse oximetry monitoring, it is imperative to use a device that will not only be accurate when the patient is well-oxygenated, but will quickly and accurately respond to sudden and/or significant oxygen desaturations.
Nonin Medical introduced its first wrist-worn pulse oximeter (WristOx® Model 3100) in 2004. Since that time, Nonin has been an industry leader in this form factor and was the first to integrate Bluetooth® technology into its wrist-worn pulse oximeters. Due to convenience of size and comfort, the company saw rapid adoption of its wrist-worn pulse oximeter in the clinical environment, particularly for continuous monitoring of oxygen saturation and heart rate during overnight studies and ambulatory tests.
The High Cost of Missed Events
In the last several years, other wrist-worn pulse oximeters have been introduced under private label by inexpensive import manufacturers. Many of these devices are low cost and available through distributors and Medicare-approved Independent Diagnostic Testing Facilities (IDTF) throughout the United States. If these devices are missing sudden and/or significant oxygen desaturations (see Graph 1), the cost to both the patient and the healthcare provider could be profound. Missed desaturation events could result in an incorrect diagnosis, delayed treatment, repeat studies, extended hospital stays or premature discharge, missed home oxygen prescriptions or repeat hospitalizations.
Nonin Medical WristOx2 Proves to be Superior in Capturing Oxygen Desaturation Events
In an independent lab test, hypoxia testing was performed on wrist-worn pulse oximeters from Nonin Medical (WristOx2® Model 3150, Plymouth, MN) and VirtuOx (VPOD, private label, Beijing Choice, Shenzhen, China).
After obtaining Institutional Review Board (IRB) approval, Clinimark Laboratories (Boulder, CO)1, an independent hypoxia laboratory, tested healthy volunteer participants by inducing hypoxia events down to the 70–85% SpO2 range. These events included concurrent induced low perfusion (one arm cooled in chilled air) and labored breathing. Nellcor brand pulse oximeters (Model N600, Medtronic, Minneapolis, MN) were used as reference monitors on both the cooled (low perfusion) and warm (normal perfusion) hands.
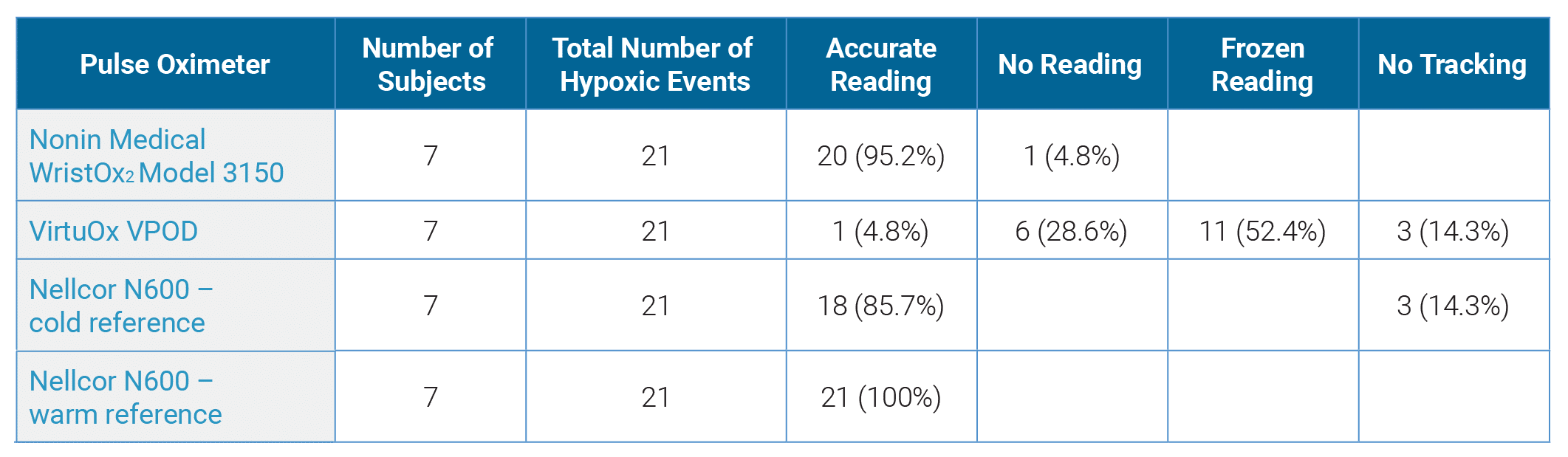
A sample size of seven subjects was tested, obtaining a total of 21 hypoxic events with nadir value below 85% SpO2. In 20 of 21 hypoxic events (95.2%), the VirtuOx VPOD pulse oximeter failed to accurately measure the oxygen desaturation, resulting in no reading (displayed value read zero), frozen reading (displayed value failed to track the desaturation) or no tracking (displayed values were >10% higher than the reference oximeters) when compared to the reference pulse oximeter. The Nonin Medical WristOx2 Model 3150 accurately measured 20 out of 21 desaturation events (95.2%) when compared to the Nellcor reference pulse oximeters. (Table 1)
In 20 of 21 hypoxic events (95.2%), the VirtuOx VPOD pulse oximeter failed to accurately measure the oxygen desaturation. The Nonin Medical WristOx2 Model 3150 accurately measured 20 out of 21 desaturation events (95.2%).
Graph 1: Example of Missed Desaturation Event by VirtuOx VPOD Wrist-Worn Pulse Oximeter
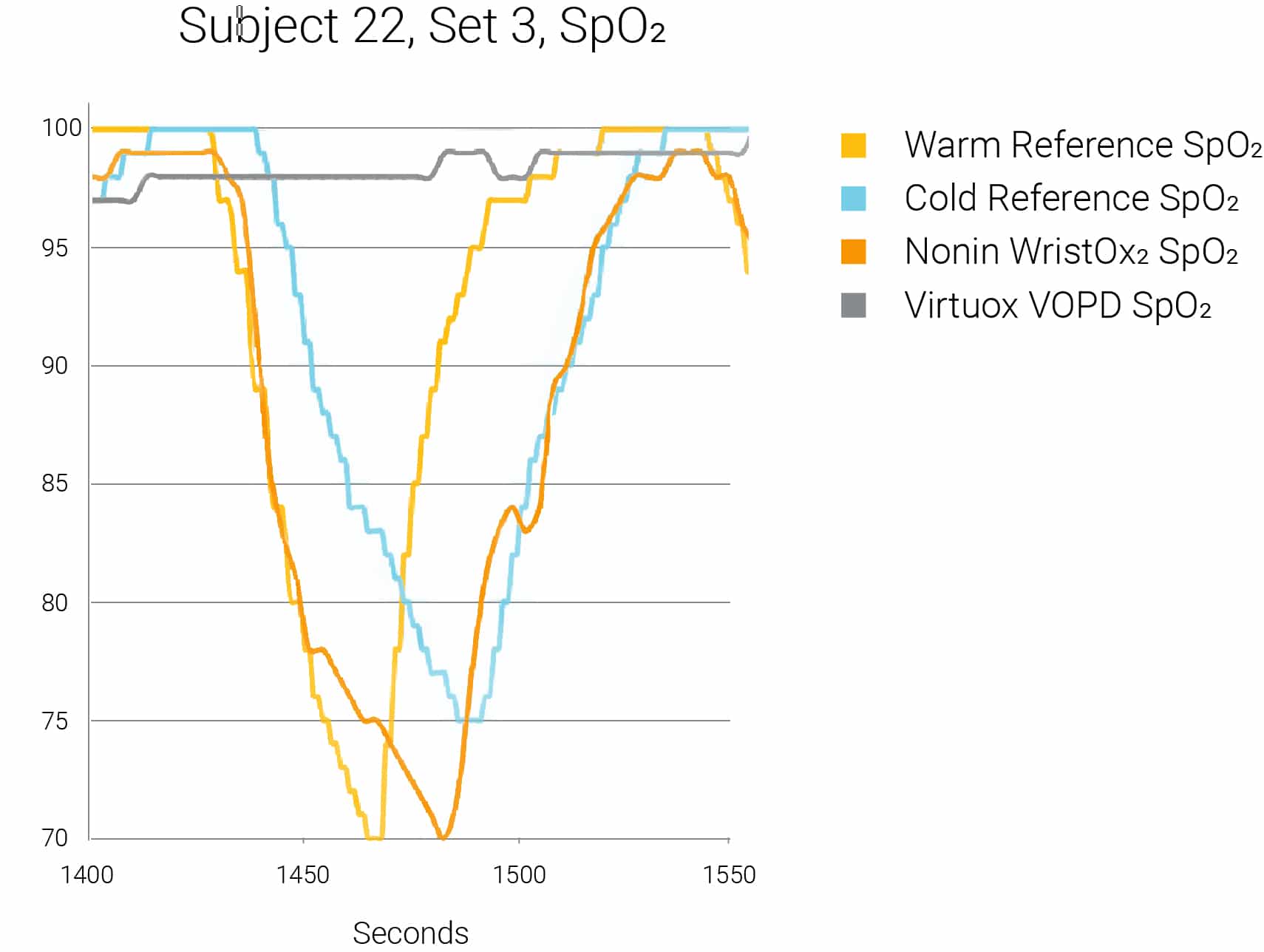
Table 1: Results by Device
Summary
This study indicates that low-quality or low-cost import pulse oximeters may not be reliable for capturing true and significant oxygen desaturations which could, in turn, lead to costly mistakes affecting both provider and patient. The low-quality or low-cost wrist oximeter tested in this study failed to track nearly all critical hypoxic events below 85% SpO2.
The Nonin Medical PureSAT® Pulse Oximetry Technology Difference
Only Nonin Medical provides proven pulse oximetry performance in the widest range of patient conditions and settings. Nonin’s clinically proven PureSAT® pulse oximetry technology uses intelligent pulse-by-pulse filtering to provide precise oximetry measurements—even in the presence of dark skin, motion, low perfusion, shortness of breath and other challenging conditions. PureSAT automatically adjusts to each patient’s condition to provide fast and reliable readings clinicians can act on.
1. P.B. Batchelder, RRT, LRCP: Fingertip Pulse Oximeter Performance in Dyspnea and Low Perfusion During Hypoxic Events. Clinimark Laboratories, Boulder Colorado.
