P.B. Batchelder, RRT, LRCP, Clinimark Laboratories, Boulder, Colorado
INTRODUCTION
The first fingertip pulse oximeter was introduced in 1995 by Nonin Medical. For approximately 13 years, it was the sole device in this form factor in which the entire pulse oximeter was housed in the finger sensor (display, engine, power supply and sensor). Due to convenience of size and, apparently few reported performance issues, it saw rapid adoption in the clinical environment. In the last eight years several new portable fingertip pulse oximeters have been introduced. Many of these devices are low cost and available over the counter. Anecdotal information gathered from clinicians indicate that some devices have poor performance which might be due to a combination of poor signal strength (lower perfusion) and labored breathing (dyspnea) resulting in inaccurate SpO2 and/or pulse rate.
“Our patients have poor peripheral perfusion (due to beta blockers), dyspnea and motion. [We have seen] slow response time, poor low perfusion performance, freezing, inconsistent performance, no reading.”
Angie Ebeling, RRT, Pulmonary Rehabilitation
North Memorial Medical Center, Robbinsdale, Minnesota
Clinimark Laboratories, an independent hypoxia lab, was asked to investigate the performance of two newer fingertip pulse oximeters and the Nonin Medical fingertip pulse oximeter during dyspnea and low perfusion. Multiple hypoxic events down to the 70–85% range were induced to verify that the pulse oximeter was able to track saturation changes during these challenging conditions. In order to do this in a repeatable, safe manner this study was conducted in a laboratory setting by creating dyspnea, low perfusion and hypoxia in healthy volunteer subjects.
METHODS
Prior to study start, Institutional Review Board approval was obtained.1 The study was registered at clinicaltrials. gov (NCT00881829) and written informed consent was obtained from each subject prior to conducting the study.
Devices Under Test
Fingertip pulse oximeters from three widely used manufacturers were studied. They were the Nonin 3230 (using the same PureLight® and PureSAT® technology as the Onyx® Vantage 9590 and Onyx® II 9550), the Beijing Choice MD300 and the Contec Medical CMS50. The latter two manufacturers distribute under several different names and are representative of many newer fingertip pulse oximeters. All devices are FDA-cleared.
Study Design
Dyspnea and low perfusion were simultaneously induced in each volunteer test subject. During the dyspneic and low perfusion periods, multiple hypoxic events down to the 70–85% SpO2 range were induced to verify that the fingertip pulse oximeter was able to track saturation changes during these challenging conditions.
Reference Devices. Two Nellcor N-600x tabletop pulse oximeters were used as reference devices. One was placed on the warm, normal perfusion hand (warm reference) and the other was on the cooled, low perfusion hand (cooled reference). Due to the dynamic nature of this trial (in which the saturation changed rapidly from ~95%, down to the 70–85% range, then back up to ~95%) this trial did not use arterial blood as a reference. This is because arterial blood reference measurement requires a stable saturation for at least one minute.2
Test Devices. The Nonin fingertip pulse oximeter was placed on one finger of the cooled test hand. One of the newer fingertip pulse oximeters was placed on the remaining finger of the cooled test hand (either a Beijing Choice ChoiceMMed MD300 or a Contec Medical CMS50).
Sample Size. The sample size was as follows:
- Beijing Choice and Nonin = 12 subjects with 54 hypoxic events
- Contec and Nonin = 13 subjects with 61 hypoxic events
Low Perfusion. The low perfusion (cooled hand) condition was created by directing chilled air (~55°F) over the right arm and right side of the body. Warm, normal perfusion conditions were created by covering the left arm and shoulder with a blanket. A warm water bottle was placed under the left hand. This allowed the left hand to remain warm with normal perfusion, while the right hand exhibited low perfusion.
Dyspnea. Dyspnea was created in healthy volunteer subjects by having the subjects hyperventilate continuously for extended periods of time. After one minute of this intense ventilatory breathing exercise, all subjects in this trial began to fatigue and exhibit labored breathing.
Hypoxic Events. Intermittent hypoxic events down to a nadir of 70–85% SpO2; (based on the reference device) were produced by administration of ~14% oxygen. The subject was asked to hyperventilate continuously during the test with the exception of short periods, at which times the subject was asked to hold their breath which created short hypoxic events down to 70–85%.
Analysis. The number of times and the duration for each of the following conditions were totaled:
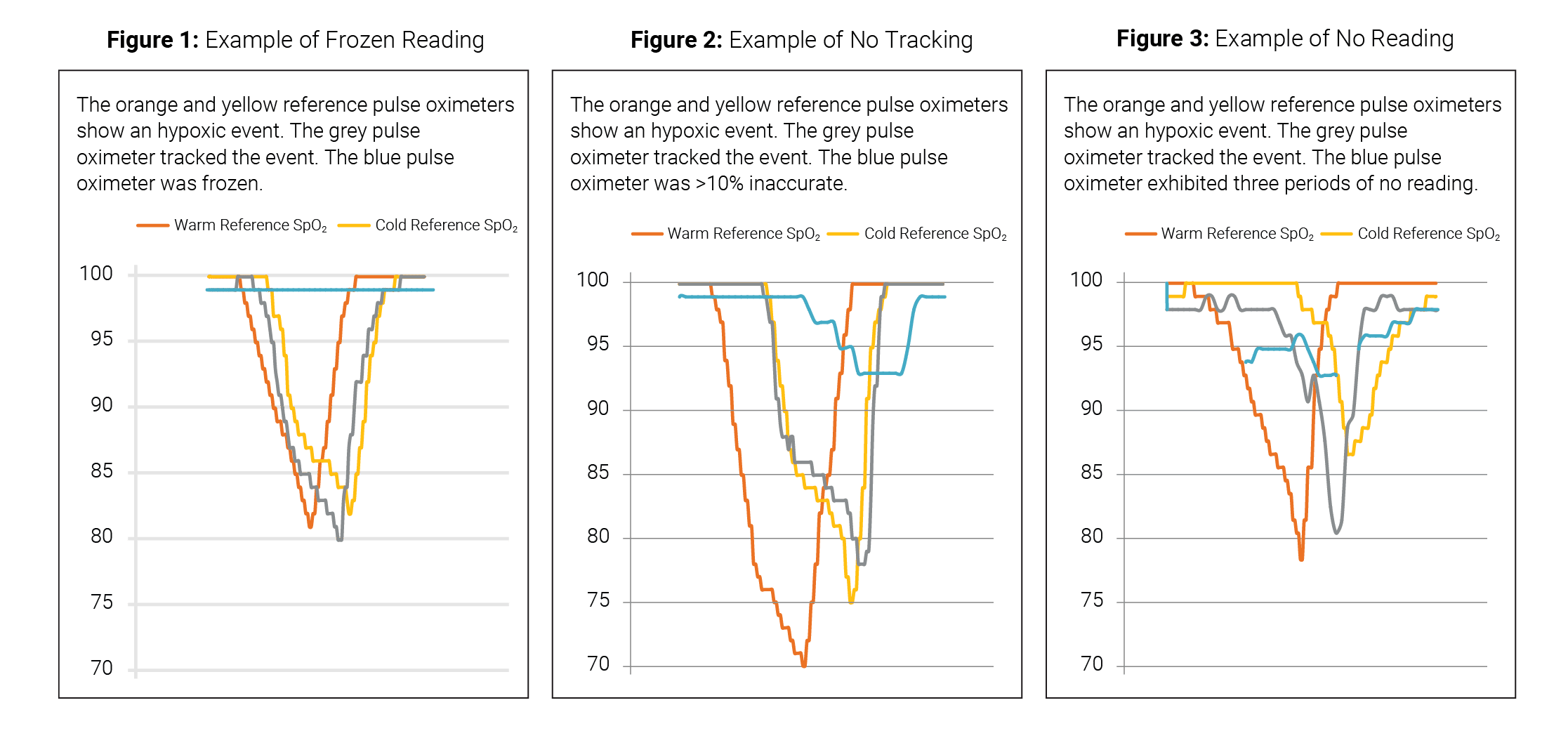
1. Frozen reading – no change in reading of the test device during a hypoxic event.
2. No tracking – test device readings displayed were >10% high compared to the reference device during a hypoxic event.
3. No reading – blank display.
In this study, performance was considered acceptable if a test pulse oximeter showed a clear pattern in tracking a hypoxic event from ~95% down to ~<85% then back up to ~95%.
“Clinicians see discrepancies in the readings when they compare the cheaper home pulse oximeter to the ones used in the hospital.”
Barbara Sherwood, BS RRT, NPS, C.O.R.E. Respiratory Services
Minneapolis, Minnesota
RESULTS
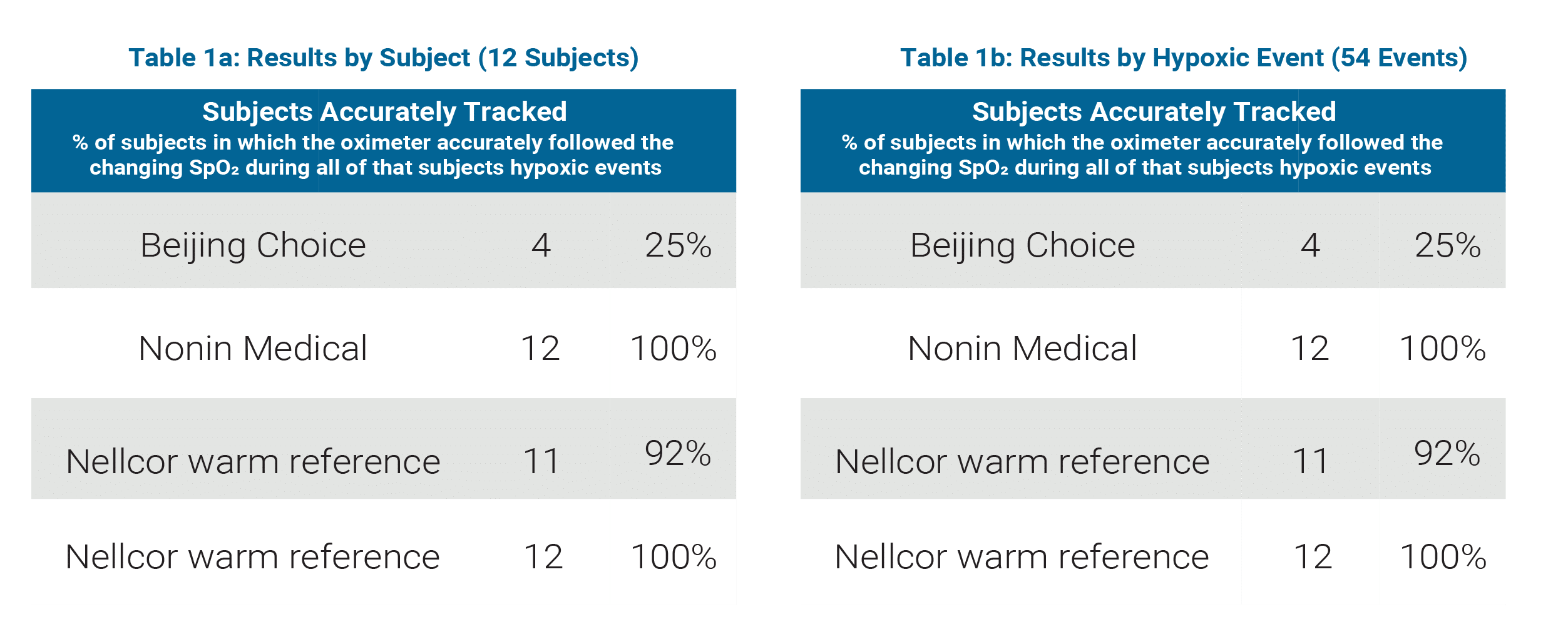
Beijing Choice / Nonin Medical Comparison
(See Table 1a and 1b)
Sample Size: 12 subjects, 54 hypoxic events
Beijing Choice
- Accurately tracked hypoxic events in 25% of subjects
- Accurately tracked 69% of all hypoxic events
Nonin Medical
- Accurately tracked hypoxic events in 100% of subjects
- Accurately tracked 100% of all hypoxic events
Nellcor normal perfusion (warm hand) reference
- Accurately tracked hypoxic events in 92% of subjects
- Accurately tracked 98% of all hypoxic events
Nellcor low perfusion (cooled hand) reference
- Accurately tracked hypoxic events in 100% of subjects
- Accurately tracked 100% of all hypoxic events
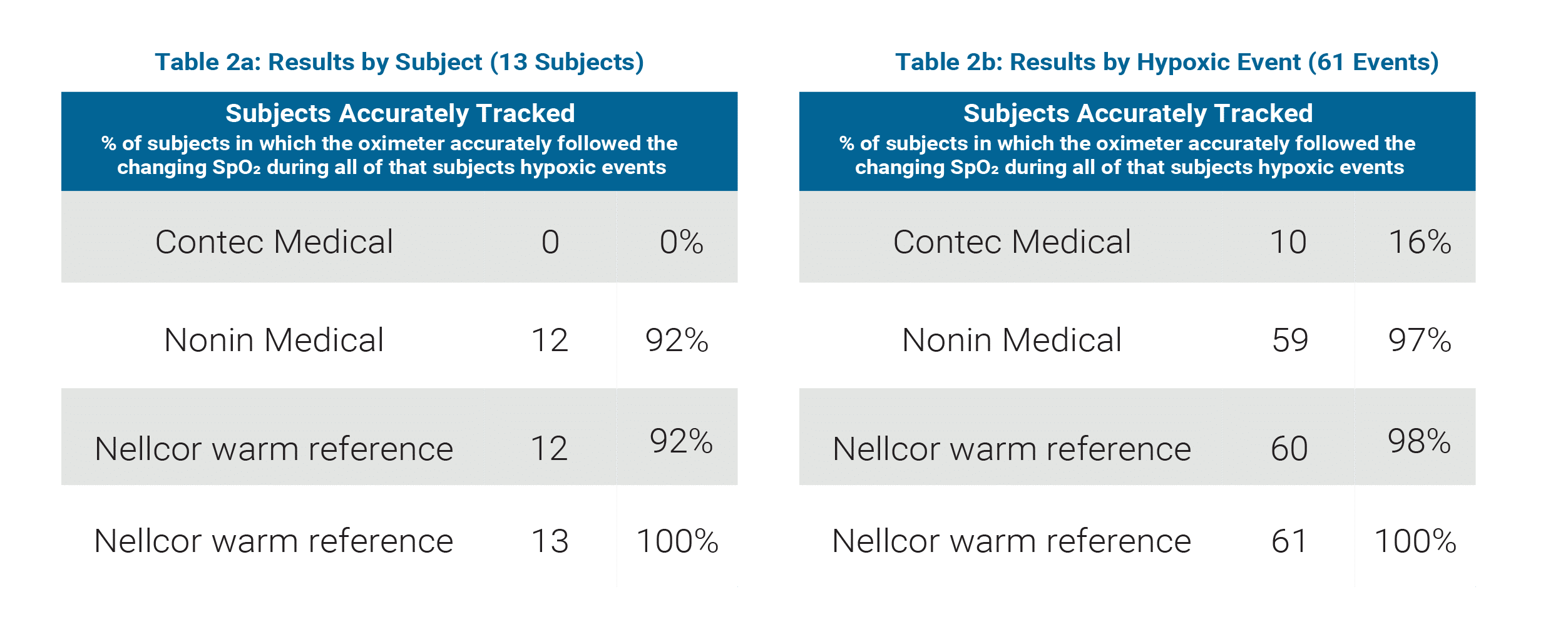
Contec Medical / Nonin Medical Comparison
(See Table 2a and 2b)
Sample Size: 13 subjects, 61 hypoxic events
Contec Medical
- Accurately tracked hypoxic events in 0% of subjects
- Accurately tracked 16% of all hypoxic events
Nonin Medical
- Accurately tracked hypoxic events in 92% of subjects
- Accurately tracked 97% of all hypoxic events
Nellcor normal perfusion (warm hand) reference
- Accurately tracked hypoxic events in 92% of subjects
- Accurately tracked 98% of all hypoxic events
Nellcor low perfusion (cooled hand) reference
- Accurately tracked hypoxic events in 100% of subjects
- Accurately tracked 100% of all hypoxic events
“Use of the cheaper finger pulse oximeters in hospitals is rare, however patients
commonly buy the least expensive option.”
Curt Merriman, RRT, CPFT
C.O.R.E. Respiratory Services, Minneapolis, Minnesota
DISCUSSION
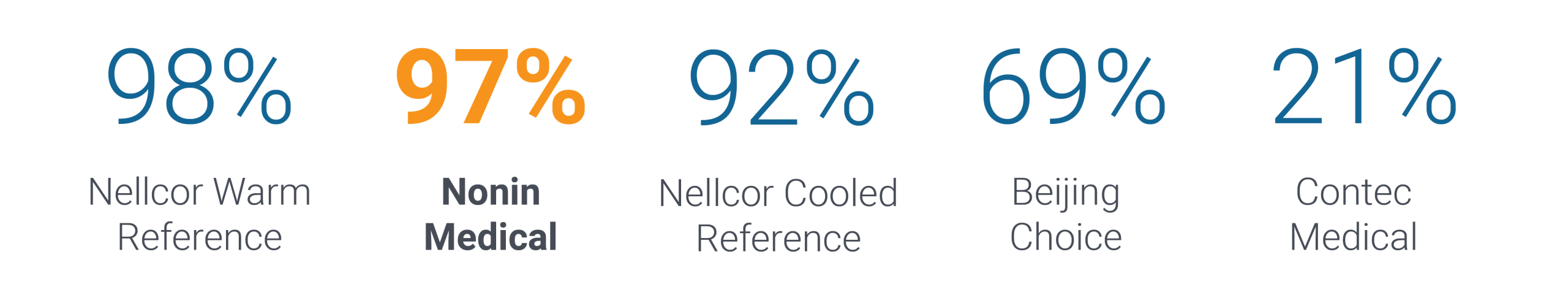
In a study that simulated conditions of dyspnea and low perfusion, fingertip pulse oximeters from Nonin Medical performed significantly better than models from Beijing Choice and Contec Medical.
Total Hypoxic Events Accurately Tracked
In the last few years fingertip pulse oximeters have been available in the U.S. as “health and wellness” devices without FDA clearance or need for a prescription. Patients have begun to purchase and use these devices to guide selfcare. And to a lesser degree, some clinicians are using these uncleared devices as well as similar inexpensive FDA cleared devices in their practice.
Concerned clinicians often conduct impromptu evaluations of these devices by putting the oximeter on themselves or on a stable patient under non-stressful conditions and comparing the values to a reference device. Performance in this type of impromptu evaluation is not representative of the dyspneic patient who may have paradoxical chest movement from labored breathing and low peripheral perfusion. Additionally, hypoxic events must be included in the testing to verify that a device is not frozen and is able to track a saturation change.
This study indicated that when performance might be most needed—during labored breathing with low perfusion—the two newer low cost oximeters tested in this study failed to track many of the hypoxic events and, instead froze the readings, read >10% high or blanked the display when the saturation was critically low.
REFERENCES
1 Avista Adventist Hospital Institutional Review Board, FWA 00011584.
2 ISO 80601-2-61 First edition 2011-04-01 Medical electrical equipment — Part 2-61: Particular requirements for basic safety and essential performance of pulse oximeter equipment.
