David B. MacLeod, F.R.C.A., Department of Anesthesiology, Duke University Medical Center, Durham, North Carolina
Introduction
Since its introduction 30 years ago, the use of pulse oximetry to continuously monitor the saturation of arterial blood led to dramatic improvements in patient safety.1,2 Like pulse oximetry, cerebral oximeters use the principles of light transmission and absorption to noninvasively measure the concentration of oxygenated and deoxygenated hemoglobin within brain tissue, thus providing a continuous measure of cerebral oxygenation. Cerebral tissue oxygenation is of particular interest, as cerebral hypoxia rapidly leads to irreversible injury. Circumstances occur, however, where tissue hypoxia can exist in the presence of appropriate arterial oxygenation.
Most oximeters consist of the same basic components: light source to deliver light of a known wavelength to the tissue; a light detector to measure the intensity of light exiting the tissue, and a process to translate changes in light intensity to a cerebral oxygen saturation value.3 Although all cerebral oximeters use these same components, each commercially available cerebral oximeter employs a unique set of wavelengths and algorithms to derive saturation values. These characteristics can impact the stability, the reliability and the accuracy of the oximeter. Despite these differences between devices, continuous, noninvasive monitoring of cerebral oxygenation is beginning to reach its long anticipated potential.
Over the last decade, numerous case reports and case series in cardiac surgery have verified the ability of cerebral oximeters to rapidly detect changes in cerebral oxygenation due to mechanical or physiological induced decreases in cerebral blood flow.4-6 These changes in cerebral oxygenation can occur even when systemic parameters (blood pressure and heart rate) are unchanged.7,8 During cardiac surgery, cerebral oxygen desaturation predicts cognitive impairment postoperatively,9 and the use of cerebral oximetry for intraoperative management has been shown to reduce morbidity and mortality.10
Prior research has validated the accuracy of the Nonin EQUANOXTM Regional Oximeter System with the three-wavelength EQUANOX ClassicTM 8000CA sensor as a reliable trend monitor. The objective of this study was to validate the EQUANOX AdvanceTM 8004CA four-wavelength sensor.
Study Device
The study device, EQUANOX 7600 with EQUANOX AdvanceTM 8004CA sensor, was developed by Nonin Medical, Inc. and measures regional blood oxygen saturation utilizing a patented technology with a dual emitter and dual detector sensor topology. Most NIRS cerebral oximeters use a single emitter and two detectors for the optical measurements. The optical measurements from the shorter path (representing extracranial blood oxygen) is subtracted from the longer path (representing intracranial and extracranial blood oxygen) to give measurements for intracranial blood oxygen.11 Any surface and shallow tissue variation between the two detector sites introduces error into the measurement.
The EQUANOX System’s dual emitter and dual receiver sensor cancels out surface and shallow tissue variations allowing for a more targeted tissue reading. EQUANOX has been shown to provide improved isolation of targeted tissue compared to single emitter systems12 and excellent repeatability when measuring regional tissue oxygenation.13 The system is completely noninvasive. The emitters are light emitting diodes (LEDs) with the following four wavelengths: 730nm, 760nm, 810nm, 880nm. Each wavelength allows for isolating a single chromophores that may vary from subject to subject. Thus, the additional wavelength in the 8004CA sensor is expected to further remove intersubject variability and pro- vide greater accuracy in assessing cerebral oxygenation status.
Methods and Materials
Testing was conducted at Duke University Human Pharmacology Lab under the direction of the author, was approved by the Duke University Institutional Review Board, and enrolled healthy, non-smoking subjects between 21–34 years of age. All 12 subjects gave written, informed consent. One subject enrolled was excluded as an arterial line could not be placed. The remaining subjects had a jugular venous catheter and a radial artery catheter placed. The venous catheter position was confirmed with a lateral skull x-ray.
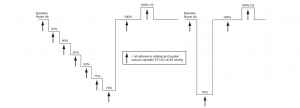
The subjects were maintained in a semi-recumbent position, and a 8004CA sensor was placed on each side of the forehead. Hypoxia was induced and managed via a dedicated facemask and breathing apparatus (RespirAct) that can precisely control blood carbon dioxide as well as deliver precise oxygen fractions to achieve hypoxemia. Subjects underwent a standard, breathdown protocol to allow for a clinically relevant range of oxygenation values. (See Figure 1 on the following page.)
Each hypoxic plateau lasted approximately 6 minutes, with a jugular bulb blood sample drawn over a 2-minute period at the end of the plateau; arterial blood samples were drawn at the beginning and the end of the jugular blood draw. Laboratory analysis (co-oximetry) was performed to determine the venous saturation (SvO2) and arterial saturation (SaO2); the two arterial samples were averaged to provide a single value for each plateau. The arteriovenous saturation (SavO2) was calculated as a 70:30 ratio: SavO2 = (0.7 x SvO2) + (0.3 x SaO2). SavO2 is a measured approximation of the cerebral oxygen saturation and was used as the reference value against which the accuracy of the rSO2 was assessed. Data collected during unstable plateaus, blood draw difficulties, co-oximeter errors or other similar circumstances were excluded.
Figure 1. Summary of the step-down plateaus
Accuracy was determined using ARMS, a common statistic for oximetry devices which estimates the agreement between a test device and an accepted reference value. ARMS consists of two components: mean bias and precision. Mean bias is the average difference between the test device value and the reference value across all observations. Precision is the scatter of possible test device values around the mean bias for a single reference value.
Results
Readings were achieved in all subjects that were attempted and every subject who underwent the breathdown was included in the analysis. A total of 119 samples from 11 subjects were analyzed. Baseline SavO2 readings breathing room air average 76.8 ± 4.8 with the 11 readings ranging from 68 to 84. The readings for rSO2 were comparable at a baseline reading of 76.6 ± 4.9 with the 11 readings with an average of 68 to 84.
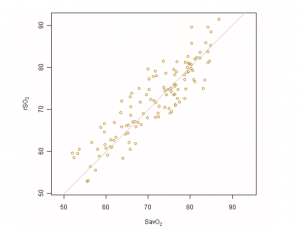
The absolute accuracy of rSO2 compared to SavO2 as measured by ARMS is 4.1%. Mean bias was 0.8 ± 4.0%. Figure 2 presents a graphical representation of the absolute accuracy. The observed rSO2 values are plotted against the measured SavO2 values. If the device had an ARMS of 0, all data would lie on the line of identity, indicating perfect agreement.
Figure 2. Agreement between rSO2 and SavO2
Conclusion
The Nonin EQUANOXTM Regional Oximeter System and EQUANOX AdvanceTM 8004CA sensor provides accurate measures of both absolute cerebral oxygen saturation and relative change in cerebral oxygen saturation across a clinically relevant range of saturations.
References
1. Tinker JH, Caplan RJ, Ward RJ, Cheney FW. Role of monitoring devices in prevention of anesthetic mishaps: a closed claims analysis. Anesthesiology. 1989;71(4):541-6.
2. Lee LA, Domino KB. The Closed Claims Project. Has it influenced anesthetic practice and outcome? Anesthesiology Clinics of North America. 2002;20(3):485- 501.
3. Wahr JA, Tremper KK, Samra S, Delph DT. Near-infrared spectroscopy: theory and applications. Journal of Cardiothoracic and Vascular Anesthesia. 1996;10(3):406-18.
4. Cordisco M, Newberger J, Shann KG, Mellas NB. Diagnosis of inadvertent cannulation of the azygos vein during cardiopulmonary bypass. J Extra Corpor Technol. 2010;42(3):235-7.
5. Fischer GW, Stone ME. Cerebral air embolism recognized by cerebral oximetry. Seminars in cardiothoracic and vascular anesthesia. 2009;13(1):56-9.
6. Han SH, Kim CS, Lim C, Kim WH. Obstruction of the superior vena cava cannula detected by desaturation of the cerebral oximeter. J Cardiothorac Vasc Anesth. 2005;19(3):420-1.
7. Casati A, Fanelli G, Pietropaoli P, et al. Continuous monitoring of cerebral oxygen saturation in elderly patients undergoing major abdominal surgery minimizes brain exposure to potential hypoxia. Anesthesia and Analgesia. 2005;101(3):740- 7, table of contents.
8. Green DW. A retrospective study of changes in cerebral oxygenation using a cerebral oximeter in older patients undergoing prolonged major abdominal surgery. Eur J Anaesthesiol. 2007;24(3):230-4.
9. Slater JP, Guarino T, Stack J, et al. Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. Ann Thorac Surg. 2009;87(1):36-44; discussion -5.
10. Murkin JM, Adams SJ, Novick RJ, Quantz M, Bainbridge D, Iglesias I, et al. Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesthesia and Analgesia. 2007;104(1):51-8.
11. Hongo K, Kobayashi S, Okudera H, Hokama M, Nakagawa F. Noninvasive cerebral optical spectroscopy: Depth-resolved measurements of cerebral haemodynamics using indocyanine green. Neurological Research. 1995;17.
12. Davie S, Grocott H. The impact of extra cranial contamination on NIRS measurements of regional cerebral oxygen saturation: a comparison of three differ- ent cerebral oximeters. Society of Cardiovascular Anesthesiologists 33rd Annual Meeting. Savannah, Georgia. May 3, 2011. Poster session.
13. Lobbestael A, Roth L, Prior M. EQUANOX Technology with dual emitter-dual detector cancels out surface and shallow tissue variation when measuring cerebral oxygenation. 2009. Data on file at Nonin Medical.
