Case Overview
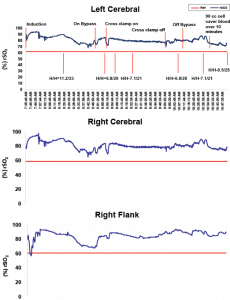
The SenSmartTM Universal Oximeter cerebral and somatic oximetry graphs shown in Figure 1 demonstrates a case in which monitoring rSO2 provided reliable data that perfusion was sufficient to avoid the use of packed red blood cells (PRBCs).
Background:
- Patient: Three year old, 13 kilogram (kg) white female
- Diagnosis: Partial atrioventricular canal (AVC), moderate mitral insufficiency and a primum atrial septal defect (ASD)
- Status: Asymptomatic, had no known drug allergies, but was taking Enalapril and had right atrial and ventricular enlargement.
- Hemoglobin and Hematocrit: Starting hemoglobin (Hg) was 12.3 gm/dL and hematocrit (Hct) 34.8%. In a patient of this size it is often possible to conduct the case without using any blood products, including the use of a crystalloid prime. This patient received a pure crystalloid prime at the commencement of cardiopulmonary bypass (CPB), primarily with Plasmalyte (Baxter Corporation, Deerfield, IL).
Operative Monitoring
Standard cardiac monitors were employed, including electrocardiogram (EKG), non- invasive blood pressure (NIBP), pulse oximeter (SaO2), end-tidal carbon dioxide (EtCO2), bladder and esophageal temperature probes, Foley bladder catheter for urine output, arterial line, and double lumen central venous line (CVL). The SenSmart Model X-100 Cerebral/Somatic Oximetry device was also used to obtain regional saturation index (rSO2)measurements. Sensors were placed over each side of the forehead to provide bilateral cerebral monitoring, and one over the right flank. Given the goal of maintaining rSO2 20 above 20% decline from baseline, those values were recorded prior to induction: right cerebral-89, left cerebral-78 and renal-79.
Figure 1: Nonin SenSmart provided reliable rSO2 data.
What the SenSmartTM System Showed
The patient underwent uneventful induction with Sevoflurane, with maintenance anesthesia also provided by Sevoflurane, as well as fentanyl and dexmedetomidine. Cooling was to 28.4° Celsius. As noted above, the goal was to perform the surgery without transfused blood products; therefore, the patient received pure crystalloid prime at the commencement of cardiopulmonary bypass (CPB), primarily with PlasmaLyte. The first arterial blood gas (ABG) was obtained roughly ten minutes after the commencement of CPB, revealing a hemoglobin of 6.8 gm/dL and hematocrit of 20%. Serial ABGs during CPB revealed the hemoglobin and hematocrit were maintained at this level. The patient was hemoconcentrated while on CPB, the last Hg/Hct (H/H) prior to separation from CPB was at 6.8/20.
After separating from CPB, blood was salvaged from the field and CPB pump with a Cell Saver and we were able to transfuse the patient 90 cc of this cell saver blood. Her H/H after this transfusion of her salvaged blood was 8.5/25.
The nadir for the NIRS values were R-78, L-76 and renal-69 immediately following commencement of CPB, which is completely expected. For the remainder of the CPB run the cerebral rSO2 values remained in the low to mid 80s and the somatic renal rSO2 also remained in this range.
Discussion
Determinants of tissue oxygenation are oxygen delivery, comprised of cardiac output, arterial oxygen saturation, and hemoglobin, and tissue oxygen extraction. The brain’s relatively high degree of oxygen extraction relative to other organs implies that it is especially at risk during periods of marginal or inadequate oxygen delivery. Thus, cerebral oxygenation can be an especially sensitive indicator of oxygen delivery, i.e., marginal oxygen delivery may result in a diminished cerebral regional saturation index (rSO2), while not affecting the somatic rSO2 until further declines in oxygen delivery occur. That said, Near-Infrared Spectroscopy (NIRS) monitoring is now commonly used to assess regional perfusion to multiple tissue beds.1,2,3 It has also been published that the use of NIRS identifies vulnerable periods during cardiac surgery and that NIRS induced interventions can improve neurological outcomes.4,5 Studies have also shown a statistically significant increase in cerebral rSO2 and somatic rSO2 after transfusion with PRBC.6
This case demonstrated that, despite the low H/H, there was adequate cerebral and somatic perfusion throughout and following the CPB run. Without the SenSmart monitor, this patient would likely have been transfused at least one unit of packed red blood cells (PRBC). Utilizing SenSmart, transfusion was confidently withheld, knowing the brain and body were receiving adequate levels of oxygen in real time, despite the severe surgically-induced anemia.
Case submitted by Christopher F. Tirotta, MD, MBA, Director, Cardiac Anesthesia, Miami Children’s Hospital, Miami FL.
References:
- Fortune PM, Wagstaff M, Petros AJ (2001) Cerebro-splanchnic oxygenation ratio (CSOR) using near infrared spectroscopy may be able to predict splanchnic ischaemia is neonates. Intensive Care Med 27:1401–1407
- Redlin M, Boettcher W, Huebler M, Berger F, Hetzer R, Koster A (2006) Detection of lower torso ischemia by near-infrared spectroscopy during cardiopulmonary bypass in a 6.8 kg infant with complex aortic anatomy.Ann Thorac Surg 52:323–325
- Weiss M, Schulz G, Teller I, Dullenkopf A, Kolarova A, Sailers H et al (2004) Tissue oxygenation monitoring during major pediatric surgery using transcutaneous liver near infrared spectroscopy. Pediatr Anesth 14:989–995
- Han HS, Kim CS, Kim SD, Bahk JH, Park YS. The effect of bloodless pump prime on cerebral oxygenation in paediatric patients. Acta Anesthesiol Scand 2004; 48:648-52
- Austin EH III, Edmonds HL Jr., Auden SM, et al. Benefit of neurophysiologic monitoring for pediatric cardiac surgery. J Thorac Cardiovasc Surg 1997;114:707-15, 717
- Bailey SM, Hendricks-Muñoz KD, Wells JT, Mally P. Packed Red Blood Cell Transfusion Increases Regional Cerebral and Splanchnic Tissue Oxygen Saturation in Anemic Symptomatic Preterm Infants. Am J Perinatol. 2010 Jan 22
