Documentation
Resources
-
Understanding Accuracy, Bias, and Variability in SpO2 Readings View
-
Clinical Bibliography: Cerebral Oximetry for Beach Chair Procedures View
-
Accuracy and Superior Performance of PureSAT and PureLight Oximetry Technologies View
-
Case Study: Partial Anomalous Pulmonary Venous Return (PAPVR) and Bicuspid Aortic Valve, Nonin Medical’s SenSmartTM Universal Oximetry View
-
Case Study: Using SenSmart Regional Oximetry to Aid in Transfusion Decisions View
-
Multi-Modality Monitoring in Carotid Surgery: The Importance of Reliable Readings View
-
Continuous Cerebral Oximetry Patient Monitoring During Post-Operative Transport Identifies Desaturation View
-
Cerebral Oxygen Desaturation Predicts Cognitive Decline and Longer Hospital Stay After Cardiac Surgery View
SenSmart Model 8204CA Specifications
rSO2 Accuracy (Arms*): 50% to 100% rSO2
| Accuracy | Right | Left | Both | Hypercapnia | Hypocapnia |
|---|---|---|---|---|---|
| Absolute | 4.1 | 3.8 | 3.9 | 5.1 | 3.3 |
| Trend | 1.9 | 3.0 | 2.5 | 3.4 | 3.8 |
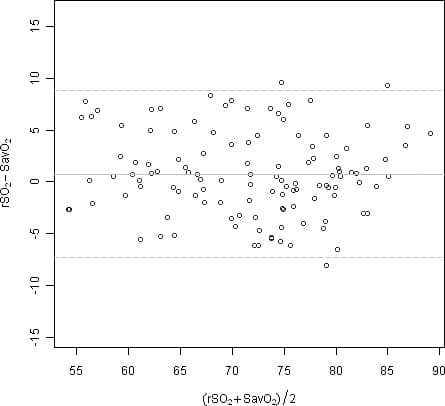
rSO2 accuracy testing was conducted during induced hypoxia studies on healthy, nonsmoking, light- to dark-skinned subjects in an independent research laboratory. The measured regional hemoglobin saturation value (rSO2) of the sensors was compared to arterial/venous hemoglobin oxygen (SavO2) value, determined from venous and arterial blood samples. The model used for blood in the brain was 70% venous and 30% arterial, which is applicable under normocapnic conditions. The venous blood was drawn from the right jugular bulb. The accuracy of the sensors in comparison to the blood gas analyzer samples measured over the rSO2 range of 45–100%. Accuracy data was calculated using the root-mean-squared (Arms value) for all subjects, per ISO 80601-2-61:2011, Medical Electrical Equipment—Particular requirements for basic safety and essential performance of pulse oximeter equipment.
Inter/Intra Sensor Repeatability Accuracy: ±2 digits (Arms*)
Temperature:
Operating: -5 °C to 40 °C (23 °F to 104 °F)
Storage/Transportation: -30 °C to 70 °C (-22 °F to 158 °F)
Device temperature will not exceed 41 °C as measured during a controlled environment test.
Humidity:
Operating: 10 % to 90 % non-condensing
Storage/Transportation: 10 % to 95 % non-condensing
* ±1 Arms encompasses 68% of the population.
Measurement Wavelengths and Output Power**
730 nanometers @ 3.0 mW maximum average power
760 nanometers @ 4.5 mW maximum average power
810 nanometers @ 3.2 mW maximum average power
880 nanometers @ 4.5 mW maximum average power
** This information is especially useful for clinicians performing photodynamic therapy.
Compliance:
This product complies with ISO 10993-1.
Sensor adhesive properties are guaranteed up to the Use By date.
Nonin
Locations
Nonin Medical Inc
(Global Headquarters)
Address
13700 1st Ave N,
Plymouth, MN 55441
Email
info@nonin.com
Toll Free (USA and Canada)
1.800.356.8874
Phone
1.763.553.9968
Nonin Medical B.V.
(Europe)
Address
Doctor Paul Janssenweg 150
5026 RH Tilburg
Netherlands
Email
info@nonin.com
