Ryan Ward, CRT, RRT Clinical Manager Respiratory Care Department, Benedictine Health Center of Minneapolis, Minneapolis, MN
Abstract: This was a prospective study designed to assess the durability and caregiver satisfaction of two models of new Durafoam Disposable pulse oximetry sensors from Nonin Medical. The study was conducted at a long-term care facility that performs continuous, multi-day respiratory monitoring for patients on ventilator care or oxygen therapy. Ten patients were each monitored continuously for at least 30 days. Sensors were replaced as was determined necessary by the caregiver. Sensor use duration and durability were assessed throughout the study. Caregivers were surveyed to assess ease of use, efficiency, fit and patient acceptance. Results: The new Nonin 6500 Durafoam Disposable Sensors designed for extended monitoring were found to be extremely durable, lasting an average 14 days, while improving caregiver efficiencies. All of the sensors lasted more than seven days and 12% lasted 21 days or more. Conversion from traditional disposable sensors to the new sensor would result in an astounding 88% reduction in sensor costs, or an estimated per day cost of just under two dollars per patient. Additional advantages included ease of use, comfort for the patient and breathability of the materials. The sensors were well-received by the caregivers and would be recommended for use by 94% of those surveyed.
Introduction
Continuous oxygen saturation monitoring is considered to be an industry standard in vital signs monitoring of patients. Conventional disposable pulse oximeters are designed to be used for continuous monitoring and are attached with a medical-grade adhesive. Applying the sensors can be time-consuming and the adhesives can be problematic for some patients who have compromised skin integrity.
Our hypothesis was that a new sensor designed for extended wear, the Durafoam Disposable Sensor by Nonin Medical, would reduce material costs associated with long-term oximetry monitoring and enhance the quality of patient care.
Methods
This was a prospective study designed to assess the durability and caregiver satisfaction of two models of the new Durafoam Disposable pulse oximetry sensors. The study was conducted at Benedictine Health Center of Minneapolis (BHCM), a long-term-acute rehabilitation facility. BHCM is 110 bed facility, with 120 clinical care employees. The patient care ratio is 6 to 1 on average throughout the facility.
The pulse oximetry sensors in this study, Nonin’s Model 6500SA and 6500MA, are single-patient use, disposable sensors indicated for continuous monitoring of adult and pediatric patients weighing more than 30 kg. The sensor is designed to be durable and provide extended, multi-day monitoring. The construction consists of a flexible wire core encased in soft foam material which conforms to the finger when applied, eliminating the need for adhesives, while providing easy removal and reapplication.

Nonin tabletop pulse oximeters (7500 or 9600) were used in conjunction with the sensors. The Nonin Onyx® II 9550 fingertip oximeters were available for spot-checking and verification of sensor placement as needed.
Ten adult patients who were ventilator-dependent or receiving oxygen therapy and required continues pulse oximetry monitoring were enrolled and monitored for at least 30 days (range: 30 to 37 days) using the new Durafoam sensors. Patients were initially fit with either the 6500SA or 6500MA sensor. Sensors were replaced as was determined necessary by the caregiver. The sensor models were alternated to allow both caregivers and patients to experience both sensors. Patients included males and female adults (age 18 or older), with varying skin color.
Date and time of sensor application and disposal and reason for sensor replacement were recorded to determine length of sensor use and durability. Caregivers were surveyed at the conclusion of the study to assess ease of use, efficiency, fit and patient acceptance.
Results
Use Duration. A total of 29 sensors were used in this study. Sensor use duration, calculated as the length in days from placement to removal, was approximately 14 days. Sensors which were replaced for reasons other than quality and/or cleanliness, such as end of study participation, were not included in the summary of sensor use duration. The use duration of the two models of sensor were similar.
All sensors lasted more than seven days and 12% lasted 21 days or more.
Sensor Lifespan at BHCM
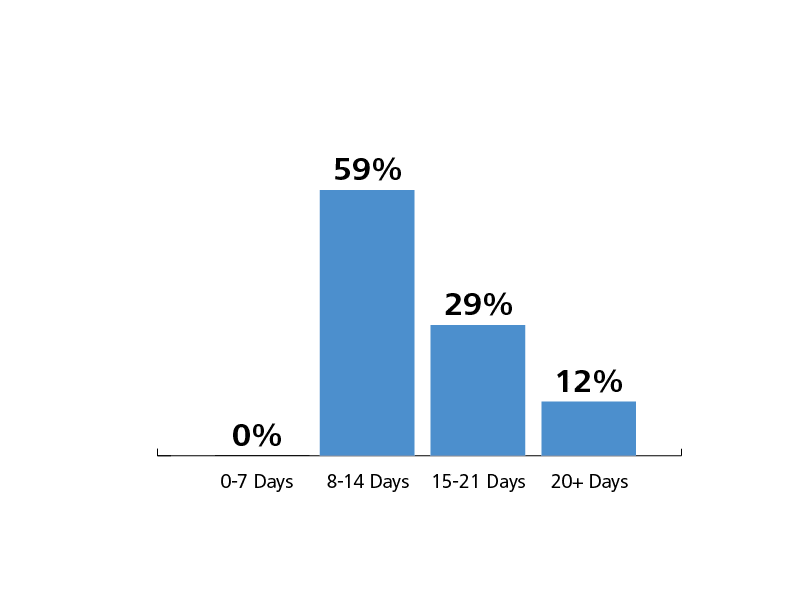
The most common reason for sensor replacement was lack of cleanliness of the sensor (n=13; 45%). Four sensors (14%) were replaced due to poor signal. The duration of use for these four sensors ranged from seven days to 23 days prior to signal degradation and replacement. The remaining 12 sensors (41%) were replaced for reasons other than durability, including end of study (n=10), patient choice/comfort (n=1) and sensor fit (n=1).
The sensor removed due to patient comfort was a 6500SA and was being used on a patient who liked to write and found the 6500SA to be cumbersome for this activity. The sensor was replaced with the 6500MA model which the subject found to be satisfactory, allowing both continuous pulse oximetry monitoring and writing activities. The patient completed the seventeen remaining days of study without further problems using a single 6500MA sensor.
The one sensor removed due to fit was a 6500SA and was being used on a patient with small, slender fingers. Due to the specific size and shape of this patient’s fingers, the 6500SA did not fit optimally. The sensor was replaced with a 6500MA which provided a secure fit and the patient completed the remaining 22 days of the study without issue.
Costs. While actual cost savings were not formally included in the study, by applying a known cost for conventional sensors and average number of sensors used per patient per day, the projected analysis indicates substantial savings. Conventional disposable sensors cost our facility $15.00 per sensor and are typically replaced daily. Thus, the average cost for adhesive disposable sensors for a typical 30-day patient stay would be $450. Based on our experience and a list-price of $27.50 for the new 6500 sensor, the cost to monitor a typical 30-day patient stay would be $55.00 on average. Replacing adhesive sensors with the Durafoam sensor would result in a $395 costs savings per patient per month, or an astounding 88% decrease in disposable sensor costs.
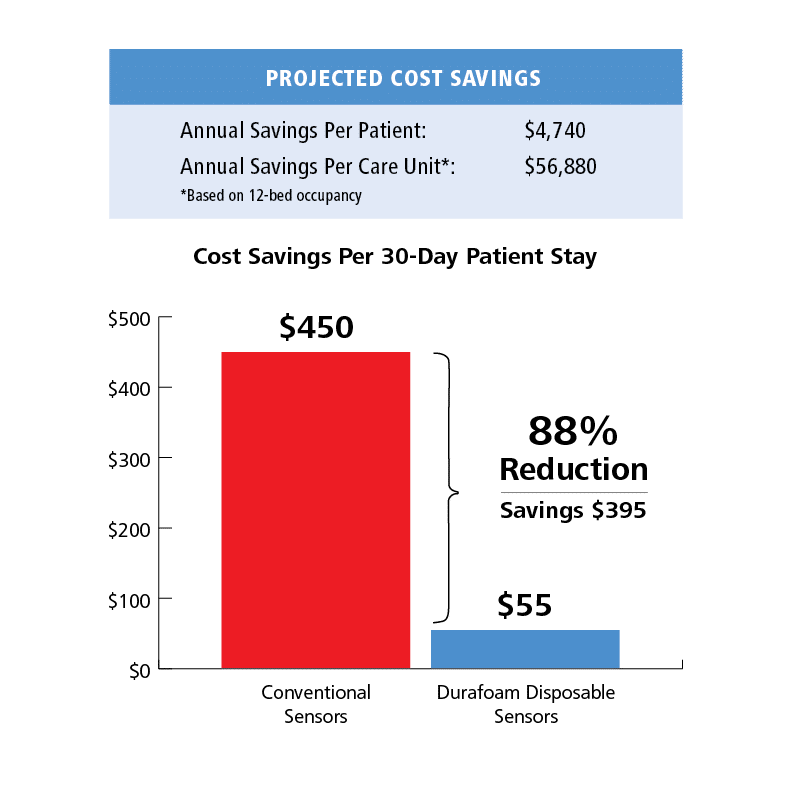
Alternatively, one can consider the per day cost of disposables for the two options. For traditional disposables that are replaced each day, individual per sensor cost equates to the daily costs, in this case, $15. For the Nonin 6500 sensors, the per day cost is calculated as the list price divided by the median use duration, resulting in an estimated per day cost of less than two dollars.
Further savings are realized in the application time. Caregivers report a significant time savings while applying the sensor due to ease of use, although the actual time savings is difficult to quantify. However, with the average replacement interval reduced from once a day to every 14 days, using a minimal figure of 2 minutes per application, the time savings on bi-weekly basis translates to an average of 28 minutes per patient.
Overall sensor cost of less than $2 per day
Ease of Use/Caregiver Acceptance. Overall, 76% of the caregivers found the sensors easier to apply than traditional disposable sensors, while only 10% indicated having any difficulty with the application. In addition, 82% indicated the extended-wear sensors offered significant time savings. Approximately three-quarters of the surveyed caregivers (78%) believed the Durafoam sensors offered a better fit than traditional disposable sensors. The only negative comments tended to be related to a preference of one model of sensor over another with regard to fit for specific size fingers.
“I like its ability to stay tight and
its perfect reading of SpO2.”
Registered Nurse
The vast majority of caregivers (94%) indicated they would recommend the Durafoam sensor to other facilities. Reasons included time savings and reliability, secure fit and easy of use.
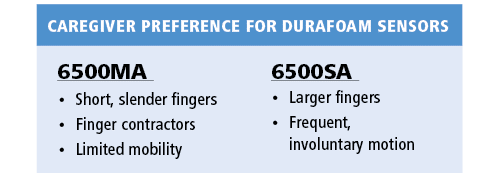
A secondary objective of the study was to assess if there were preferences for one sensor model over the other. In our experience, both models were acceptable and are needed based on individual patient characteristics. In general, model preference was dependent on the patients’ mobility and finger size. Overall the 6500MA was preferred for fit and ease of use while the 6500SA offered more stability and was better suited for larger hands.
Discussion
Continuous oxygen saturation monitoring through pulse oximetry offers an elevated level of patient care as opposed to spot-checking patients. Nonin’s Durafoam Disposable sensor designed for extended wear proved to be extremely durable, offering continuous pulse oximetry monitoring while significantly reducing supply costs and increasing caregiver efficiency. Multiple advantages over traditional adhesive disposable sensors were identified, including ease of use, durability and comfort. The new sensors were able to meet the needs of a diverse range of patients in this study.
The most notable advantage is the product’s durability, which results in extended use over multiple days and thus results in significant cost savings. Based on our experience, we projected an 88% reduction in disposable costs. This was based on our current disposable sensor price of $15 which may be greater than larger facilities and 14-day duration of use for the new sensor. Even with a lower comparative disposable sensor cost of $11 per sensor ($330/30 day stay) and a more conservative estimate of a 7-day duration of use for the new sensor ($110/30 day stay), conversion to the new sensor would result in a 66% cost reduction. In our experience, all sensors lasted at least seven days and so this duration of use estimate is quite low compared to the results we experienced.
When examined as a per day sensor cost for an individual patient, the new sensor cost was less than two dollars per monitored day. The shortest duration of use that we experienced for sensors that were replaced due to durability/cleanliness was seven days. Even in this scenario, the cost of disposables for monitoring is less than four dollars per day; more than 50% below published averages of approximately nine dollars per sensor (idata research 2010).
The Durafoam sensor material was well-received and reported to be comfortable by the patients. The foam material is breathable and soft against the patient skin. The material is similar to that which you would find on athletic ski goggles and thus by design allows for air flow around the sensor. As a result, caregivers noted less sweating and odor. So, although the sensor is used for multiple days, odor was not an issue. Caregivers compared the odor after seven days of using the Durafoam sensor to two hours of adhesive disposable sensors. An RN with experience in an acute care facility noted that the “comfort and softness would be seen as positive in an ICU setting”.
The design form of the sensor eliminates the need for adhesives, which is likely to have also contributed to the cleanliness. More importantly, however, the lack of adhesive has the advantage of reducing potential skin irritations and breakdown. There was no incidence of skin integrity compromise throughout the study and the caregivers reported less need to reposition the sensors due to skin irritation. Included in the study was a patient with multiple known allergies. Although this patient had previously had reactions to adhesives in traditional sensors, the patient was included in the study and completed the full 30 days of monitoring with no adverse reaction to the materials reported.
The 6500 Durafoam sensors were found to be easy to apply and reposition compared to adhesive sensors. Training for the use of the new sensors consisted of a brief 15 minute in-service session and all caregivers were able to quickly position the sensors appropriately. The new sensor is designed with a guide-hole to assist in proper finger alignment during initial application. The construction then allows the sensor to be form-fit to the individual’s unique finger size and shape, providing custom fit. Of even greater benefit is the ease in repositioning and reapplying the sensor following removal for hygiene or other needs. Whereas adhesive sensors can often lose their ability to adhere, the 6500 Durafoam sensors were able to be removed and reapplied quickly and repeatedly over time. Additionally, caregivers noted the simplicity of applying the new sensor when wearing gloves, when the patients hands were slightly damp (following bathing or sweating)—clinical scenarios which are all especially challenging when using conventionally adhesive sensors.
The sensors are labeled for use in adults and pediatrics above 30 kilograms. Although our study consisted of adults, we did have some fairly small, frail elderly patients in the study population. We found the sensors were able to accommodate their fingers adequately, although there was a preference by caregivers to use the 6500MA for patients with more slender, petite fingers for a more secure fit. Similarly, even the largest of patient’s hands were able to be accommodated by one of the two sensor models. Overall, the two models compliment a wide range of individual needs based on finger size and shape, mobility, patient positioning/movement needs and clinical complications such as contractures. Given the two models of sensors, we were always able to find an appropriate sensor for the patients in our study.
Conclusion
The new Nonin 6500 Durafoam Disposable Sensors designed for extended monitoring were found to be extremely durable, lasting an average 14 days, while improving caregiver efficiencies. All of the sensors lasted more than seven days and 12% lasted 21 days or more. Conversion from traditional disposable sensors to the new sensor would result in an astounding 88% reduction in sensor costs, or an estimated per day cost of just under two dollars per patient. Additionally, advantages included ease of use, comfort for the patient and breathability of the materials. The sensors were well-received by the caregivers and would be recommended for us by 94% of those surveyed.
This project was supported by Nonin Medical, Inc. Minneapolis-based Nonin Medical, the inventor of fingertip pulse oximetry, designs and manufactures noninvasive physiological monitoring solutions. Nonin distributes its products—including pulse oximeters, capnographs, sensors, regional oximeters and software—to clinicians and consumers in more than 125 countries. The company has more than 100 OEM partners worldwide. Nonin’s industry-leading capabilities in signal processing and sensor design provide the foundation for delivering accurate monitoring data in the widest range of patient populations and settings. Since certifying the world’s first Continua™-Certified product in 2009, the company continues to drive global connectivity standards and add low power, interoperable solutions across its product line. For more information, visit www.nonin.com.
