See the Technology Difference
Accuracy You Can Act On
A study comparing the accuracy in motion of a Nonin Medical pulse oximeter with PureSAT® technology and a competitor pulse oximeter was conducted at a leading hypoxia research laboratory. Accuracy was determined using an industry standard breathe-down protocol of induced hypoxia in thirteen subjects. SpO2 values are compared to the gold standard which is CO-oximetry analysis of arterial blood samples. Motion was generated using a mechanical fixture with tapping and rubbing.
Nonin oximetry with PureSAT technology was found to have superior accuracy. Nonin precision was ±2.1; competitor technology precision was ±14.4.1
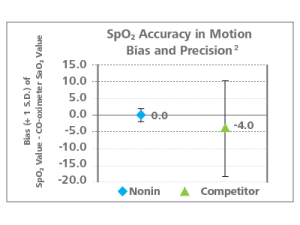
Accuracy You Can Count On
The Nonin finger pulse oximeter with PureSAT technology accurately tracked the subjects’ desaturation and had an outstanding correlation with the CO-oximeter. In addition, Nonin’s finger oximeter was able to read through motion. The competitor oximeter gave false high and false low SpO2 readings as compared to the CO-oximeter reference values. The competitor oximeter was unable to read through motion and provided no readings during motion as indicated by gaps in the green chart line.
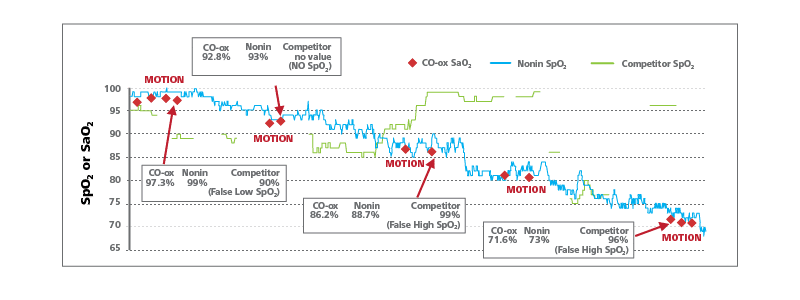
Oximeters tested: Nonin finger pulse oximeter with PureSAT technology (made in USA); competitor finger pulse oximeter manufactured by Beijing Choice Electronic Technology Co., Ltd. (made in China).
- https://www.nonin.com/resource/accuracy-and-superior-performance-of-puresat-and-purelight-oximetry-technologies/
- https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pulse-oximeters-premarket-notification-submissions-510ks-guidance-industry-and-food-and-drug
