Documentation
Resources
-
Understanding Accuracy, Bias, and Variability in SpO2 Readings View
-
Accuracy and Superior Performance of PureSAT and PureLight Oximetry Technologies View
-
Fingertip Pulse Oximeter Performance in Dyspnea and Low Perfusion During Hypoxic Events View
-
Accurate Oximetry Monitoring – See the Technology Difference View
Onyx Vantage 9590 Specifications
Oxygen Saturation Display Range: 0% to 100% SpO2
Pulse Rate Display Range: 18 to 321 beats per minute (BPM)
Declared Accuracy:
The tables below show Arms values measured using the Onyx Vantage 9590 in a clinical study in non-motion conditions.
Accuracy Summary by Decade – Finger and Thumb
| Decade | Oxygen Saturation (Arms) |
|---|---|
| 70 – 80% | ±2 |
| 80 – 90% | ±2 |
| 90 – 100% | ±2 |
| 70 – 100% | ±2 |
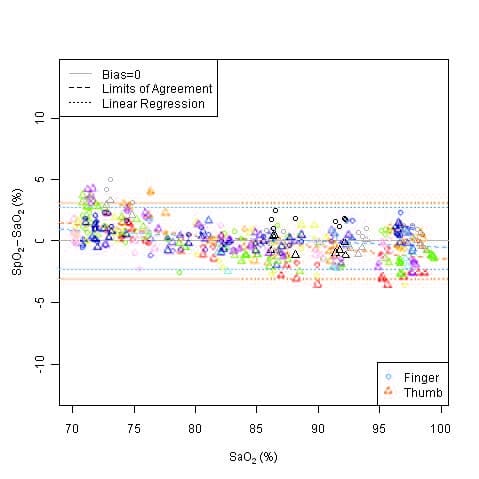
This graph shows plots of the error (SpO2 – SaO2) by SaO2 using the 9590 with a linear regression fit and upper 95% and lower 95% limits of agreement. Each sample data point is identified by subject from a clinical study in non-motion conditions.
Accuracy Summary by Decade – Toe
| Decade | Oxygen Saturation (Arms) |
|---|---|
| 70 – 80% | ±2 |
| 80 – 90% | ±3 |
| 90 – 100% | ±3 |
| 70 – 100% | ±3 |
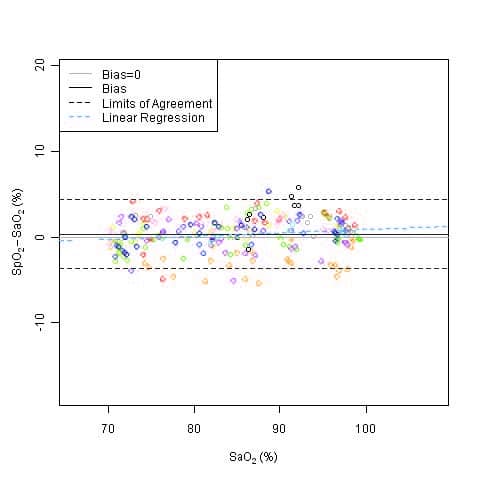
This graph shows plots of the error (SpO2 – SaO2) by SaO2 using the 9590 with a linear regression fit and upper 95% and lower 95% limits of agreement. Each sample data point is identified by subject from a clinical study using toes in non-motion conditions.
SpO2 Low Perfusion Accuracy (Arms*) 70 to 100% ±2 digits
Pulse Rate Declared Accuracy Range (Arms*): 20 to 250 BPM ±3 digits
Low Perfusion Pulse Rate Declared Accuracy Range (Arms*): 40 to 240 BPM ±3 digits
Measurement Wavelengths and Output Power**:
Red: 660 nanometers @ 0.8 mW maximum average
Infrared: 910 nanometers @ 1.2 mW maximum average
Temperature:
Operating: 23 °F to 104 °F / -5 °C to 40 °C
Storage/Transportation: -40 °F to 158 °F / -40 °C to 70 °C
Time (from storage) for monitor to be ready for its intended use: 3 minutes to warm from -40 °C to -5 °C
5 minutes to cool from 70 °C to 40 °C
Humidity:
Operating: 10% to 90% non-condensing
Storage/Transportation: 10% to 95% non-condensing
Altitude:
Operating: Up to 13,123 feet / 4,000 meters
Hyperbaric Pressure: Up to 4 atmospheres
Battery Life:
Operating: Approximately 6,000 spot checks or 36 hours of continuous operation
using new alkaline batteries.
Storage: 12 months
Classifications per ANSI/AAMI ES60601-1 / CAN/CSA-C22.2 No. 60601-1:
Degree of Protection: Type BF-Applied Part
Enclosure Degree of Ingress Protection: IP32
Mode of Operation: Continuous
This product complies with ISO 10993-1, Biological evaluation of medical devices – Part 1: Evaluation and testing.
This device is not made with natural rubber latex.
*± 1 Arms represents approximately 68% of measurements at zero bias.
**This information is especially useful for clinicians performing photodynamic therapy.
8339-001-05
Frequently Asked Questions
Can this unit be used on adults and pediatrics?
It is intended for spot-checking or attended-care monitoring of adult or pediatric patients with a finger width between 0.3 – 1.0 inch (0.8 – 2.5 cm) thick.
How long can I wear this unit?
The Onyx is not recommended where motion is expected or for relatively longterm monitoring (e.g. more than 30 minutes).
Nonin
Locations
Nonin Medical Inc
(Global Headquarters)
Address
13700 1st Ave N,
Plymouth, MN 55441
Email
info@nonin.com
Toll Free (USA and Canada)
1.800.356.8874
Phone
1.763.553.9968
Nonin Medical B.V.
(Europe)
Address
Doctor Paul Janssenweg 150
5026 RH Tilburg
Netherlands
Email
info@nonin.com
